Ergoamides
A request that this article title be changed to Lysergamides is under discussion. Please do not move this article until the discussion is closed. |

Ergoamides[1][2] (syn. lysergamides) are amides of lysergic acid (LA). The simplest ergoamide is ergine, which is also known as lysergic acid amide (LSA) or as lysergamide. The ergoamides include numerous serotonin and dopamine receptor agonists, most notably lysergic acid diethylamide (LSD). Ergoamides have an embedded tryptamine structure and, as such, some, like LSD, induce psychedelic effects similarly to psychedelic tryptamines.[3][4][5][6][7][8][9][10][11][12][13][14][15][excessive citations] Ergoamides also have a phenethylamine (e.g., mescaline) pharmacophore.[16]
Use and effects
[edit]The dosages, potencies, durations, and effects of ergoamides have been reviewed by Alexander Shulgin.[17][18][19][20][21] They have also been reviewed by Albert Hofmann,[22] David E. Nichols,[23] and other researchers.[24][25][26][27][28][29]
List of lysergamides
[edit]| Structure | Name (synonyms) | CAS # | R1 | R6 | R2 | R3 | Other |
|---|---|---|---|---|---|---|---|
 |
Ergine (lysergic acid amide, lysergamide) | 478-94-4 | H | CH3 | H | H | - |
 |
DAM-57 (lysergic acid dimethylamide) | 4238-84-0 | H | CH3 | CH3 | CH3 | - |
 |
Ergometrine (ergonovine; lysergic acid propanolamide) | 60-79-7 | H | CH3 | CH(CH3)CH2OH | H | - |
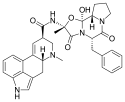 |
Ergotamine (an ergopeptine) | 113-15-5 | H | CH3 | -- | C17H18N2O4 | - |
 |
Methylergometrine (methylergonovine; lysergic acid butanolamide) | 113-42-8 | H | CH3 | CH(CH2CH3)CH2OH | H | - |
 |
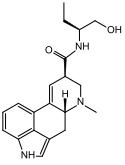
Methysergide (1-methyl-lysergic acid butanolamide) | 361-37-5 | CH3 | CH3 | CH(CH2CH3)CH2OH | H | - |
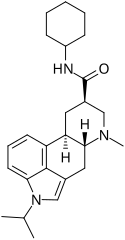 |
Amesergide (9,10-dihydro-11-isopropyllysergic acid cyclohexylamide) | 121588-75-8 | CH(CH3)2 | CH3 | C6H11 | H | - |
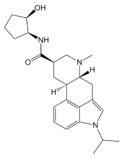 |
LY-215840 (1-isopropyl-9,10-dihydro-N-(2-hydroxycyclopent-anyl)lysergamide) | 137328-52-0 | CH(CH3)2 | CH3 | C5H8OH | H | - |
 |
Cabergoline (N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)-6-(prop-2-en-1-yl)-9,10-dihydrolysergamide) | 81409-90-7 | H | H2C=CH-CH2 | CONHCH2CH3 | CH2CH2CH2N(CH3)2 | - |
 |
LAE-32 (lysergic acid ethylamide) | 478-99-9 | H | CH3 | CH2CH3 | H | - |
 |
LAiP (lysergic acid isopropylamide) | H | CH3 | CH(CH3)2 | H | - | |
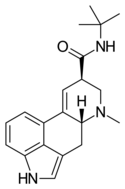 |
LAtB (lysergic acid tert-butylamide) | H | CH3 | C(CH3)3 | H | - | |
 |
LAcB (lysergic acid cyclobutylamide) | H | CH3 | (CH2)4 | H | - | |
 |
Cepentil (lysergic acid cyclopentylamide) | H | CH3 | (CH2)5 | H | - | |
 |
LSB (lysergic acid 2-butylamide) | 137765-82-3 | H | CH3 | CH(CH3)CH2CH3 | H | - |
 |
LSP (lysergic acid 3-pentylamide) | H | CH3 | CH(CH2CH3)CH2CH3 | H | - | |
| DPL (lysergic acid dipropylamide) | H | CH3 | CH2CH2CH3 | CH2CH2CH3 | - | ||
 |
DAL (lysergic acid diallylamide) | H | CH3 | H2C=CH-CH2 | H2C=CH-CH2 | - | |
 |
MIPLA (lysergic acid methylisopropylamide) | 100768-08-9 | H | CH3 | CH(CH3)2 | CH3 | - |
 |
EIPLA (lysergic acid ethylisopropylamide) | H | CH3 | CH(CH3)2 | CH2CH3 | - | |
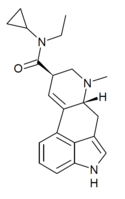 |
ECPLA (lysergic acid ethylcyclopropylamide) | H | CH3 | C3H5 | CH2CH3 | - | |
 |
ETFELA (lysergic acid N-ethyl-N-(2,2,2-trifluoroethyl)amide) | H | CH3 | CH2CF3 | CH2CH3 | - | |
 |
LAMPA (lysergic acid methylpropylamide) | 40158-98-3 | H | CH3 | CH2CH2CH3 | CH3 | - |
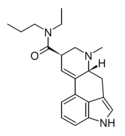 |
EPLA (lysergic acid ethylpropylamide) | H | CH2CH3 | CH2CH2CH3 | CH3 | - | |
 |
LSD (lysergic acid diethylamide; LAD) | 50-37-3 | H | CH3 | CH2CH3 | CH2CH3 | - |
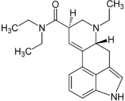 |
ETH-LAD (6-ethyl-6-nor-LSD) | 65527-62-0 | H | CH2CH3 | CH2CH3 | CH2CH3 | - |
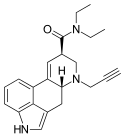 |
PARGY-LAD (6-propynyl-6-nor-LSD) | H | HC≡C−CH2 | CH2CH3 | CH2CH3 | - | |
 |
AL-LAD (6-allyl-6-nor-LSD) | 65527-61-9 | H | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - |
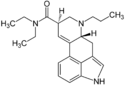 |
PRO-LAD (6-propyl-6-nor-LSD) | 65527-63-1 | H | CH2CH2CH3 | CH2CH3 | CH2CH3 | - |
 |
IP-LAD (6-isopropyl-6-nor-LSD) | H | CH(CH3)2 | CH2CH3 | CH2CH3 | - | |
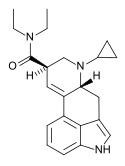 |
CYP-LAD (6-cyclopropyl-6-nor-LSD) | H | C3H5 | CH2CH3 | CH2CH3 | - | |
 |
BU-LAD (6-butyl-6-nor-LSD) | 96930-87-9 | H | CH2CH2CH2CH3 | CH2CH3 | CH2CH3 | - |
 |
FLUORETH-LAD (6-fluoroethyl-6-nor-LSD) | H | CH2CH2F | CH2CH3 | CH2CH3 | - | |
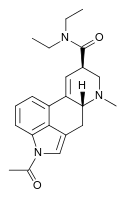 |
ALD-52 (1-acetyl-LSD) | 3270-02-8 | COCH3 | CH3 | CH2CH3 | CH2CH3 | - |
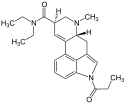 |
1P-LSD (1-propionyl-LSD) | 2349358-81-0 | COCH2CH3 | CH3 | CH2CH3 | CH2CH3 | - |
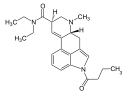 |
1B-LSD (1-butanoyl-LSD) | 2349376-12-9 | COCH2CH2CH3 | CH3 | CH2CH3 | CH2CH3 | - |
 |
1V-LSD (1-valeryl-LSD) | CO(CH2)3CH3 | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1H-LSD (1-hexanoyl-LSD) | CO(CH2)4CH3 | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1DD-LSD (1-dodecanoyl-LSD) | CO(CH2)10CH3 | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1cP-LSD (1-cyclopropylmethanoyl-LSD) | COC3H5 | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1D-LSD (1-(1,2-dimethylcyclobutane-1-carbonyl)-LSD) | COC4H5(CH3)2 | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1T-LSD (1-(thiophene-2-carbonyl)-LSD) | COC4H3S | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1S-LSD (1-(3-(trimethylsilyl)propionyl)-LSD) | CO(CH2)2Si(CH3)3 | CH3 | CH2CH3 | CH2CH3 | - | |
 |
1P-AL-LAD (1-propionyl-6-allyl-6-nor-LSD) | COCH2CH3 | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - | |
 |
1cP-AL-LAD (1-cyclopropylmethanoyl-6-allyl-6-nor-LSD) | COC3H5 | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - | |
 |
1T-AL-LAD (1-(2-thienoyl)-6-allyl-6-nor-LSD)[30] | COC4H3S | H2C=CH-CH2 | CH2CH3 | CH2CH3 | - | |
 |
1P-ETH-LAD (1-propionyl-6-ethyl-6-nor-LSD) | COCH2CH3 | CH2CH3 | CH2CH3 | CH2CH3 | - | |
 |
1P-MIPLA (1-propionyl-lysergic acid methylisopropylamide) | COCH2CH3 | CH3 | CH(CH3)2 | CH3 | - | |
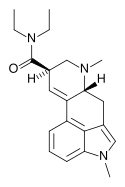 |
MLD-41 (1-methyl-LSD) | 4238-85-1 | CH3 | CH3 | CH2CH3 | CH2CH3 | - |
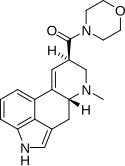 |
LSM-775 (lysergic acid morpholide) | 4314-63-0 | H | CH3 | CH2CH2-O-CH2CH2 | - | |
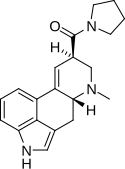 |
LPD-824 (lysergic acid pyrrolidide) | 2385-87-7 | H | CH3 | (CH2)4 | - | |
 |
LSD-Pip (lysergic acid piperidide) | 50485-23-9 | H | CH3 | (CH2)5 | - | |
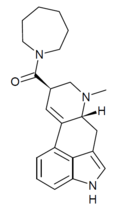 |
LSD-Azapane (lysergic acid azapane) | H | CH3 | (CH2)6 | - | ||
 |
Lysergic acid 2,4-dimethylazetidide (LA-SS-Az, LSZ) | 470666-31-0 | H | CH3 | CH2(CHCH3)2CH2 | - | |
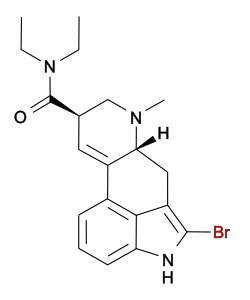 |
2-Bromo-LSD (BOL-148; bromolysergide) | 478-84-2 | H | CH3 | CH2CH3 | CH2CH3 | 2-Br |
 |
1P-BOL-148 (1-propionyl-2-bromo-LSD) | COCH2CH3 | CH3 | CH2CH3 | CH2CH3 | 2-Br | |
 |
12-Hydroxy-LSD (12-OH-LSD) | 60573-89-9 | H | CH3 | CH2CH3 | CH2CH3 | 12-OH |
 |
12-Methoxy-LSD (12-MeO-LSD) | 50484-99-6 | H | CH3 | CH2CH3 | CH2CH3 | 12-OMe |
 |
13-Fluoro-LSD[31] | H | CH3 | CH2CH3 | CH2CH3 | 13-F | |
 |
14-Hydroxy-LSD[32] | H | CH3 | CH2CH3 | CH2CH3 | 14-OH | |
Related compounds
[edit]Simplified or partial lysergamides
[edit]Simplified or "partial" ergolines and lysergamides in which one or more carbon atoms or bonds in the ergoline ring system have been removed are known.[33][34][35]
| Structure | Name | Chemical name | CAS # |
|---|---|---|---|
 |
NDTDI | N,N-diethyl-3-(methyl(1,3,4,5-tetrahydrobenzo[cd]indol-4-yl)amino)propanamide | |
 |
RU-28251[36][37] | N,N-dipropyl-1,3,4,5-tetrahydrobenzo[cd]indol-4-amine | |
 |
Bay R 1531 (LY-197206) | 1,3,4,5-tetrahydro-6-methoxy-N,N-dipropyl-benz[cd]indol-4-amine | 98770-54-8 |
 |
LY-293284 | (4R)-6-acetyl-4-(di-n-propylamino)-1,3,4,5-tetrahydrobenz[c,d]indole | 141318-62-9 |
 |
LY-178210 | 4-(dipropylamino)-1,3,4,5-tetrahydrobenzo[cd]indole-6-carboxamide | 114943-19-0 |
 |
RU-28306 | N,N-dimethyl-1,3,4,5-tetrahydrobenzo[cd]indol-4-amine | 73625-11-3 |
 |
RU-27849 | 1,3,4,5-tetrahydrobenzo[c,d]indol-4-amine | 77963-70-3 |
 |
N-DEAOP-NMT | N-(3-diethylamino-3-oxopropyl)-N-methyltryptamine | |
 |
N-DEAOP-NET | N-(3-diethylamino-3-oxopropyl)-N-ethyl-5-tryptamine | |
 |
N-DEAOP-5-MeO-NMT | N-(3-diethylamino-3-oxopropyl)-N-methyl-5-methoxytryptamine | |
 |
N-DEAOP-5-MeO-NET | N-(3-diethylamino-3-oxopropyl)-N-ethyl-5-methoxytryptamine | |
 |
10,11-Seco-LSD ("[0124]")[31] | 9,10-didehydro-N,N-diethyl-6-methyl-10,11-secoergoline-8β-carboxamide | |
 |
CT-5252 | methyl-12-bromo-8,9-didehydro-2,3β-dihydro-6-methyl-10,11-secoergoline-8-carboxylate | |
 |
10,11-Secoergoline | 3-(piperidin-2-ylmethyl)-1H-indole | 5275-05-8 |
 |
DEIMDHPCA | (3R)-N,N-diethyl-5-(1H-indol-4-yl)-1-methyl-3,6-dihydro-2H-pyridine-3-carboxamide | 2640392-28-3 |
 |
WXVL_BT0793LQ2118 | 6-fluoro-4-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-1H-indole | |
 |
"Compound 163" or "VIII"[25][38] | N,N-diethyl-4-methyl-2,3,4,4a,5,6-hexahydrobenzo(f)quinoline-2-carboxamide | |
 |
MPT | N-methyl-N-propyltryptamine | 850032-72-3 |
See also
[edit]- Substituted tryptamine
- Substituted phenethylamine
- Ergoline
- Bromocriptine
- Descarboxylysergic acid
- Fumigaclavine C
- Hydergine
- Lisuride
- Pergolide
References
[edit]- ^ Jamieson CS, Misa J, Tang Y, Billingsley JM (2021-04-29). "Biosynthesis and synthetic biology of psychoactive natural products". Chemical Society Reviews. 50 (12): 6950–7008. doi:10.1039/D1CS00065A. ISSN 0306-0012. PMC 8217322. PMID 33908526.
"There are three main ergot alkaloid classes, clavines, ergoamides (lysergamides), and ergopeptides, with 3 belonging to the ergoamide class." 2.5 Lysergic acid and LSD, p. 6970 - ^ Wong G, Lim LR, Tan YQ, Go MK, Bell DJ, Freemont PS, et al. (2022-02-07). "Reconstituting the complete biosynthesis of D-lysergic acid in yeast". Nature Communications. 13 (1): 712. Bibcode:2022NatCo..13..712W. doi:10.1038/s41467-022-28386-6. ISSN 2041-1723. PMC 8821704. PMID 35132076.
"The ergot alkaloids are broadly classified into three groups—the clavines, ergoamides, and the ergopeptines, all of which are distinguished by the different modifications appended to the core ergoline structure." Results and discussion / Biosynthetic resolution of the ergot alkaloid pathway - ^ US patent 2997470, Pioch RP, "Lysergic Acid Amides", published 1956-03-05, issued 1961-08-22
- ^ Hoffman AJ, Nichols DE (September 1985). "Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives". Journal of Medicinal Chemistry. 28 (9): 1252–1255. doi:10.1021/jm00147a022. PMID 4032428.
- ^ Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE (March 1994). "Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives". Pharmacology, Biochemistry, and Behavior. 47 (3): 667–673. doi:10.1016/0091-3057(94)90172-4. PMID 8208787. S2CID 16490010.
- ^ Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB (April 1995). "LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors". Psychopharmacology. 118 (4): 401–409. doi:10.1007/BF02245940. PMID 7568626. S2CID 21484356.
- ^ Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (September 2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". Journal of Medicinal Chemistry. 45 (19): 4344–4349. doi:10.1021/jm020153s. PMID 12213075.
- ^ Schiff PL (October 2006). "Ergot and its alkaloids". American Journal of Pharmaceutical Education. 70 (5): 98. doi:10.5688/aj700598 (inactive 2024-11-22). PMC 1637017. PMID 17149427.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
- ^ Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, et al. (September 2016). "Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD)". Drug Testing and Analysis. 8 (9): 891–902. doi:10.1002/dta.1884. PMC 4829483. PMID 26456305.
- ^ Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, et al. (January 2017). "Return of the lysergamides. Part II: Analytical and behavioural characterization of N6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2'S,4'S)-lysergic acid 2,4-dimethylazetidide (LSZ)". Drug Testing and Analysis. 9 (1): 38–50. doi:10.1002/dta.1985. PMC 5411264. PMID 27265891.
- ^ Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Stratford A, et al. (October 2017). "Return of the lysergamides. Part III: Analytical characterization of N6 -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD)". Drug Testing and Analysis. 9 (10): 1641–1649. doi:10.1002/dta.2196. PMC 6230477. PMID 28342178.
- ^ Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, et al. (February 2018). "Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775)". Drug Testing and Analysis. 10 (2): 310–322. doi:10.1002/dta.2222. PMC 6230476. PMID 28585392.
- ^ Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Dowling G, et al. (August 2019). "Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B-LSD)". Drug Testing and Analysis. 11 (8): 1122–1133. doi:10.1002/dta.2613. PMC 6899222. PMID 31083768.
- ^ Halberstadt AL, Klein LM, Chatha M, Valenzuela LB, Stratford A, Wallach J, et al. (February 2019). "Pharmacological characterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide (ECPLA)". Psychopharmacology. 236 (2): 799–808. doi:10.1007/s00213-018-5055-9. PMC 6848745. PMID 30298278.
- ^ Lee K, Poudel YB, Glinkerman CM, Boger DL (2015). "Total synthesis of dihydrolysergic acid and dihydrolysergol: development of a divergent synthetic strategy applicable to rapid assembly of D-ring analogs". Tetrahedron. 71 (35): 5897–5905. doi:10.1016/j.tet.2015.05.093.
"Embedded in the structures of the ergot alkaloids are conformationally-restricted variants of the phenethylamine pharmacophores of both dopamine and related biogenic amines as well as that of serotonin." - ^ Shulgin AT (2003). "Basic Pharmacology and Effects". In Laing RR (ed.). Hallucinogens: A Forensic Drug Handbook. Forensic Drug Handbook Series. Elsevier Science. pp. 67–137. ISBN 978-0-12-433951-4. Retrieved 1 February 2025.
- ^ Jacob P, Shulgin AT (1994). "Structure-activity relationships of the classic hallucinogens and their analogs" (PDF). NIDA Res Monogr. 146: 74–91. PMID 8742795.
- ^ Shulgin AT (1982). "Chemistry of Psychotomimetics". In Hoffmeister F, Stille G (eds.). Psychotropic Agents, Part III: Alcohol and Psychotomimetics, Psychotropic Effects of Central Acting Drugs. Berlin: Springer Berlin Heidelberg. pp. 3–29. doi:10.1007/978-3-642-67770-0_1. ISBN 978-3-642-67772-4. OCLC 8130916.
- ^ Alexander T. Shulgin (1980). "Hallucinogens". In Burger A, Wolf ME (eds.). Burger's Medicinal Chemistry. Vol. 3 (4 ed.). New York: Wiley. pp. 1109–1137. ISBN 978-0-471-01572-7. OCLC 219960627.
- ^ Alexander T. Shulgin, Ann Shulgin (1997). "#26. LSD-25 ACID; LYSERGIDE; D-LYSERGIC ACID DIETHYLAMIDE; METH-LAD; D-LYSERGAMIDE, N,N-DIETHYL; N,N-DIETHYL-D-LYSERGAMIDE; 9,10-DIDEHYDRO-N,N-DIETHYL-6-METHYLERGOLINE-8b-CARBOXAMIDE". TiHKAL: The Continuation (1st ed.). Berkeley, CA: Transform Press. pp. 490–499. ISBN 978-0-9630096-9-2. OCLC 38503252.
The second major location of variations in the structure of LSD has been in the nature of the alkyl groups on the amide nitrogen atom. Some of these are Sandoz syntheses, some are from other research groups, and a few of them are found in nature. Some of these have been studied in man, and some have not. A few of the original clutch of Sandoz compounds have both 1-substituents and amide alkyl (R) group variations: [...]
- ^ Hofmann A (June 1959). "Psychotomimetic Drugs: Chemical and Pharmacological Aspects" (PDF). Acta Physiol Pharmacol Neerl. 8: 240–258. PMID 13852489.
- ^ Nichols DE (2018). Chemistry and Structure-Activity Relationships of Psychedelics. Current Topics in Behavioral Neurosciences. Vol. 36. pp. 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. PMID 28401524.
- ^ Rutschmann J, Stadler PA (1978). "Chemical Background". In Berde B, Schild HO (eds.). Ergot Alkaloids and Related Compounds. Handbook of Experimental Pharmacology (HEP). Vol. 49. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 29–85. doi:10.1007/978-3-642-66775-6_2. ISBN 978-3-642-66777-0.
- ^ a b Mangner TJ (1978). Potential Psychotomimetic Antagonists. N,n -diethyl-1-methyl-3-aryl-1, 2, 5, 6-tetrahydropyridine-5-carboxamides (Ph.D. thesis). University of Michigan. doi:10.7302/11268.
The overall design of the sequence is based on one described by Horii154 for the synthesis of a compound very closely related to the desired 112 -- the hexahydrobenzoquinolone 163, for which, incidentally, no pharmacological details were given. [...] 154. Z. Horii, T. Kurihara, S. Yamamoto and I. Ninomiya, Chem. Pharm. Bull. (Tokyo), 15, 1641 (1967).
- ^ Fanchamps A (1978). "Some Compounds With Hallucinogenic Activity". In Berde B, Schild HO (eds.). Ergot Alkaloids and Related Compounds. Handbook of Experimental Pharmacology (HEP). Vol. 49. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 567–614. doi:10.1007/978-3-642-66775-6_8. ISBN 978-3-642-66777-0.
- ^ Rothlin E (March 1957). "Lysergic acid diethylamide and related substances". Ann N Y Acad Sci. 66 (3): 668–676. doi:10.1111/j.1749-6632.1957.tb40756.x. PMID 13425249.
- ^ Hoffer A (1965). "D-Lysergic Acid Diethylamide (LSD): A Review of its Present Status". Clin Pharmacol Ther. 6: 183–255. doi:10.1002/cpt196562183. PMID 14288188.
- ^ Isbell H, Miner EJ, Logan CR (1959). "Relationships of psychotomimetic to anti-serotonin potencies of congeners of lysergic acid diethylamide (LSD-25)". Psychopharmacologia. 1: 20–28. doi:10.1007/BF00408108. PMID 14405872.
- ^ Okada Y, Segawa H, Yamamuro T, Kuwayama K, Tsujikawa K, Kanamori T, et al. (June 2024). "Synthesis and analytical characterization of 1-(2-thienoyl)-6-allyl-nor-d-lysergic acid diethylamide (1T-AL-LAD)". Drug Testing and Analysis. doi:10.1002/dta.3747. PMID 38922764.
- ^ a b WO 2021076572, David E. Olson; Lee Dunlap & Florence Wagner et al., "Ergoline-like compounds for promoting neural plasticity", published 22 April 2021, assigned to Delix Therapeutics, Inc. and The Regents of the University of California
- ^ Libânio Osório Marta RF (August 2019). "Metabolism of lysergic acid diethylamide (LSD): an update". Drug Metabolism Reviews. 51 (3): 378–387. doi:10.1080/03602532.2019.1638931. PMID 31266388.
- ^ Shulgin AT (1976). "Psychotomimetic Agents". In Gordon M (ed.). Psychopharmacological Agents: Use, Misuse and Abuse. Medicinal Chemistry: A Series of Monographs. Vol. 4. Academic Press. p. 59–146. doi:10.1016/b978-0-12-290559-9.50011-9. ISBN 978-0-12-290559-9.
The largest number of structural analogs of LSD that have been prepared involve the opening of one or more of the rings of the parent lysergic acid system. The compounds with the piperidine ring (ring D) opened [see (I)] are encountered as natural products in the several Convolvulaceae discussed in Section II,B on ololiuqui. The opening of ring C (by cleavage of the 10-11 bond to the indole "4 position") results in a series of N-α-disubstituted tryptamines. Additionally, analogs are known with the indolic nitrogen replaced with sulfur (benzothiophenes) and with an aliphatic chain (tetralins). A recent review covers this chemistry (Campaigne and Knapp, 1971), but there is apparently no human psychopharmacology as yet known.
- ^ Nichols DE (May 1973). Potential Psychotomimetics: Bromomethoxyamphetamines and Structural Congeners of Lysergic Acid (Thesis). University of Iowa. pp. 23–23. OCLC 1194694085.
- ^ Campaigne E, Knapp DR (June 1971). "Structural analogs of lysergic acid". J Pharm Sci. 60 (6): 809–814. doi:10.1002/jps.2600600602. PMID 4942861.
- ^ Euvrard C, Ferland L, Fortin M, Oberlander C, Labrie F, Boissier JR (1981). "Dopaminergic activity of some simplified ergoline derivatives". Drug Development Research. 1 (2): 151–161. doi:10.1002/ddr.430010208. ISSN 0272-4391.
- ^ "N,N-dipropyl-1,3,4,5-tetrahydrobenzo[cd]indol-4-amine". PubChem. Retrieved 21 March 2025.
- ^ Horii Z, Kurihara T, Yamamoto S, Ninomiya I (November 1967). "Studies on ergot alkaloids and related compounds. XIV. Synthesis of N-alkyl-4-methyl-2,3,4,4a,5,6-hexahydrobenzo[f]quinoline-2-carboxamides and stereochemistry of diethyl 4-methyl-1-oxo-1,2,3,4,4a,5,6,10b-octahydro-benzo[f]quinoline-2,2-dicarboxylate". Chem Pharm Bull (Tokyo). 15 (11): 1641–1650. doi:10.1248/cpb.15.1641. PMID 5583819.
Our previous report1) introduced the synthesis of a potent oxytocic ethyl 4-methyl-2,3,4,4a,5,6-hexahydrobenzo[f]quinoline-2-carboxylate (VII). Recently, Ohta and his coworkers also prepared V] by a convenient method starting from the Mannich product (II). However, they did not deal with the stereochemistry of the compounds involved. In the course of work searching for compounds with potent activity related to lysergic acid, we now wish to describe two routes of preparations of diethyl-, n-butyl- and 2-hydroxyisopropylamide derivatives of VI, which can be regarded as LSD25 analogs lacking only a pyrrole ring, and also discuss the stereochemistry of this series of compounds. [...] Of these amide derivatives, the diethylamide (VIII) was also prepared by an alternative route as follows. [...] Experimental. [...] N,N-Diethyl-4-methyl-2,3,4,4a,5,6-hexahydrobenzo(f)quinoline-2-carboxamide (VIII)—[...]
